Secondary Pharma – Control System


Project Summary
GlaxoSmithKline is one of the world’s leading research-based pharmaceutical and healthcare companies. Due to the growing demand for traceability in all pharmaceutical manufacturing process’s and the increasing regulations being specified by the European Union and countries worldwide, GlaxoSmithKline needed a state of the art manufacturing facility to handle all of these issues.
New System Requirements:
- Full batch execution system.
- Recipe Management system.
- Batch, alarm and event reporting system.
- User interface audit system.
- Complete automatic and manual control of production sequences.
- Complete 21 CFR Part 11
Technical Challenges:
- Integration of multiple 3rd party technologies with site-standardised Siemens Automation .
- Design of a completely versatile and flexible step based recipe management system.
- Design of a batch, alarm and event reporting system.
- Integration with the onsite MES System.
- Incorporating 21 CFR Part 11 compliance into entire system, using electronic signatures authenticated against the company domain.
- Design system validation documents and test cases. Coordination of the system validation.
Customer Benefits:
CG Controls designed and delivered a new solution that allowed site management convert existing product production methodology from a primarily manual control environment with paper-based compliance and traceability records to a fully compliant automated paperless system, in addition to providing plant operational staff with a highly-flexible, full featured and user-friendly control system interface.
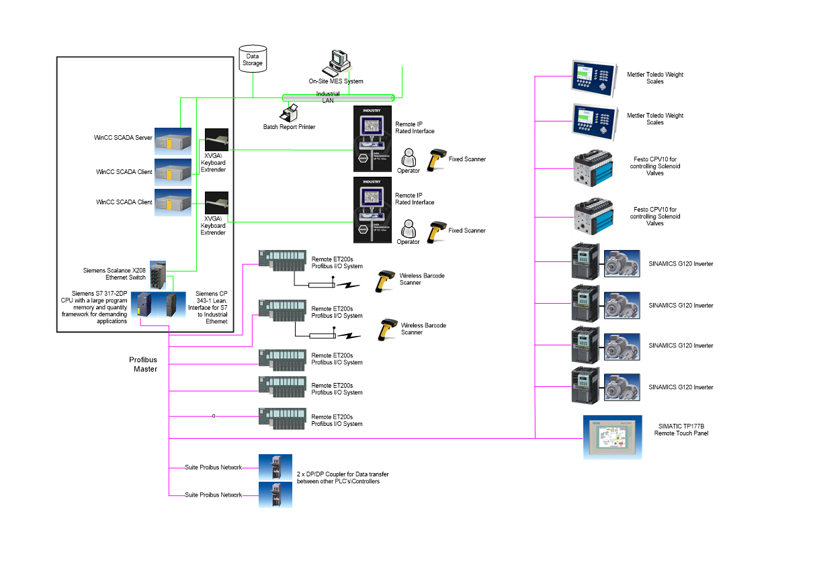
System Architecture